What Are Wiped Film Molecular Distillation’s Pharma Applications?
Wiped Film Molecular Distillation has become an invaluable technology in pharmaceutical manufacturing, thanks to its ability to purify heat-sensitive and high-value compounds with exceptional precision. In the pharmaceutical industry, where product purity, safety, and efficacy are critical, traditional separation methods often fall short—especially when dealing with delicate molecules that degrade under high heat or complex mixtures requiring strict impurity control. Wiped Film Molecular Distillation addresses these challenges by operating under high vacuum and low temperatures, making it ideal for a range of pharmaceutical applications. This guide explores the key ways Wiped Film Molecular Distillation is used in pharma, highlighting its benefits and impact on drug development and production.
Purification of Active Pharmaceutical Ingredients (APIs)
Active Pharmaceutical Ingredients (APIs) are the core components of drugs that deliver therapeutic effects. Their purity directly affects a drug’s safety and efficacy, as even small impurities can cause side effects or reduce potency. Wiped Film Molecular Distillation plays a vital role in purifying APIs, especially those that are heat-sensitive or difficult to separate using other methods.
Many APIs, such as peptides, proteins, and certain small-molecule drugs, are sensitive to high temperatures. Traditional distillation or chromatography methods may expose these molecules to heat or harsh solvents, leading to degradation. Wiped Film Molecular Distillation, however, uses high vacuum to lower boiling points, allowing APIs to be purified at temperatures 50–100°C lower than those required in conventional processes. This gentle approach preserves the API’s chemical structure and biological activity.
Additionally, Wiped Film Molecular Distillation effectively removes impurities like residual solvents, byproducts, or isomers that have similar chemical properties to the target API. For example, in the production of anti-cancer drugs or antibiotics, where purity levels must often exceed 99.9%, this technology ensures that even trace contaminants are eliminated, meeting strict regulatory standards set by agencies like the FDA or EMA.
Separation of Chiral Compounds
Chiral compounds are molecules with mirror-image structures (enantiomers), where one form may have therapeutic effects while the other is inactive or even harmful. Separating these enantiomers is critical in pharmaceutical manufacturing, as regulators require strict control over chiral purity. Wiped Film Molecular Distillation is uniquely suited for this task, even when enantiomers have very similar boiling points.
In traditional methods, chiral separation often relies on expensive chiral chromatography columns or toxic solvents, which are costly and environmentally unfriendly. Wiped Film Molecular Distillation, by contrast, separates chiral compounds based on subtle differences in volatility and molecular weight, achieved under high vacuum. The thin film created by the wiping mechanism ensures that even small differences in vaporization rates are amplified, allowing for efficient separation.
This application is particularly valuable in the production of drugs for central nervous system disorders, cardiovascular diseases, and pain management, where chiral purity is essential for efficacy and safety. Wiped Film Molecular Distillation reduces reliance on solvents, lowers costs, and improves the efficiency of chiral separation processes.
Removal of Residual Solvents
Pharmaceutical manufacturing often uses organic solvents to dissolve or synthesize APIs, but residual solvents can remain in the final product. These solvents are regulated tightly, as some are toxic or carcinogenic. Wiped Film Molecular Distillation is highly effective at removing residual solvents, ensuring compliance with international standards (such as ICH Q3C guidelines).
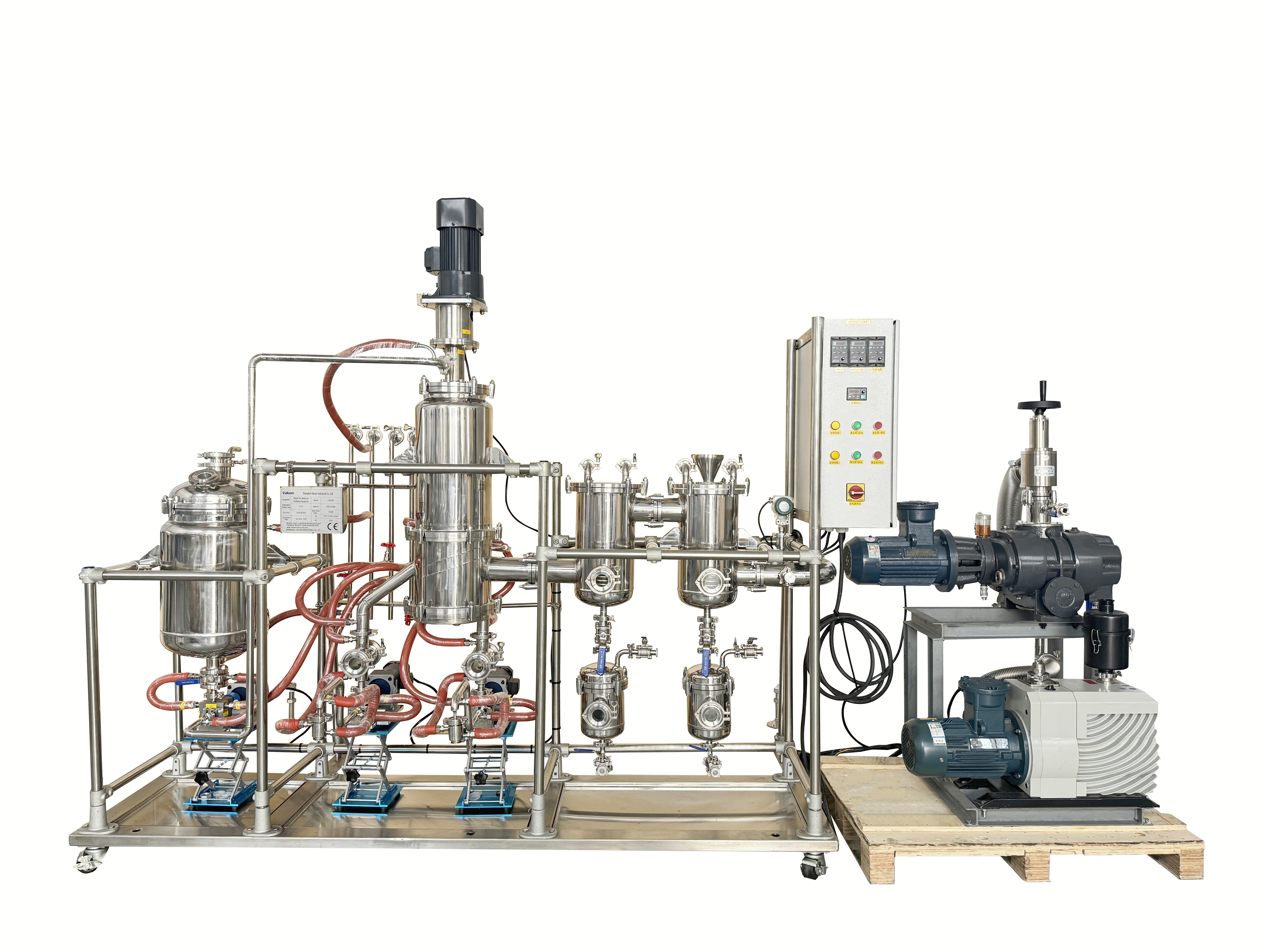
Solvents like methanol, acetone, or dichloromethane have lower boiling points than most APIs, making them ideal targets for distillation. Under high vacuum, Wiped Film Molecular Distillation vaporizes these solvents at low temperatures, separating them from the API without causing thermal damage. The short path between the heated film and condenser ensures that solvents are removed efficiently, with minimal loss of the target compound.
This process is crucial for parenteral drugs (injected medications) and oral formulations, where even trace solvents can pose health risks. Wiped Film Molecular Distillation achieves solvent levels well below regulatory limits (often less than 10 ppm), ensuring patient safety and compliance.
Concentration of Heat-Sensitive Bioactive Compounds
Many pharmaceuticals are derived from natural sources, such as plants, bacteria, or fungi, which contain bioactive compounds like alkaloids, terpenes, or polysaccharides. These compounds are often heat-sensitive, meaning traditional concentration methods (like evaporation) can destroy their therapeutic properties. Wiped Film Molecular Distillation offers a gentle way to concentrate these bioactive compounds while preserving their integrity.
For example, in the production of herbal medicines or natural product-based drugs, Wiped Film Molecular Distillation can concentrate active components (such as antioxidants or anti-inflammatory agents) by removing water or volatile impurities. The low-temperature, high-vacuum environment prevents degradation, ensuring the compounds retain their biological activity.
This application is also valuable in the production of vaccines and biopharmaceuticals, where proteins or peptides must be concentrated without denaturation. Wiped Film Molecular Distillation’s ability to handle delicate biological molecules makes it a key technology in biopharma manufacturing.
Purification of Lipid-Based Drug Delivery Systems
Lipid-based drug delivery systems (LBDDS), such as liposomes, emulsions, and lipid nanoparticles, are widely used to improve the solubility, stability, and bioavailability of APIs—especially hydrophobic drugs that are difficult to formulate. Purifying these lipid-based systems requires gentle processing to avoid disrupting their structure, making Wiped Film Molecular Distillation an ideal choice.
Lipids are often heat-sensitive and can oxidize or degrade at high temperatures. Wiped Film Molecular Distillation purifies LBDDS by removing excess lipids, unencapsulated APIs, or surfactants under low-temperature conditions. The thin film and short path design ensure that lipid structures remain intact, preserving the delivery system’s functionality.
In mRNA vaccines, for example, lipid nanoparticles protect the fragile mRNA molecules and facilitate their delivery into cells. Wiped Film Molecular Distillation helps purify these nanoparticles by removing unreacted lipids and solvents, ensuring the vaccine’s stability and efficacy. This application highlights the technology’s role in advancing modern pharmaceutical formulations.
Deodorization and Decontamination of Pharmaceutical Excipients
Excipients are inactive ingredients in drugs that improve stability, texture, or delivery (e.g., binders, fillers, or lubricants). While inactive, excipients must be pure to avoid contaminating the final product or causing adverse reactions. Wiped Film Molecular Distillation is used to deodorize and decontaminate excipients like oils, waxes, or polymers, ensuring they meet pharmaceutical standards.
For example, vegetable oils used as excipients may contain impurities or off-odors from their natural sources. Wiped Film Molecular Distillation removes these impurities by vaporizing volatile contaminants at low temperatures, leaving the oil pure and odor-free. Similarly, polymers used in drug coatings can be purified to remove residual monomers or additives that could leach into the drug.
This application ensures that excipients are safe and compatible with APIs, reducing the risk of batch failures or regulatory issues.
Scale-Up from Lab to Production
Pharmaceutical development involves scaling processes from small lab batches to large-scale production. Wiped Film Molecular Distillation supports this scale-up seamlessly, making it a valuable tool throughout the drug development lifecycle.
Lab-scale Wiped Film Molecular Distillation systems allow researchers to optimize purification parameters (such as vacuum level, temperature, and wiper speed) for new APIs or formulations. These parameters can then be transferred to pilot-scale and industrial-scale systems, ensuring consistent results across production volumes. This scalability reduces the risk of process failures during scale-up, which is critical for meeting tight development timelines and regulatory requirements.
Whether producing grams of a new API for clinical trials or tons for commercial distribution, Wiped Film Molecular Distillation maintains the same level of purity and efficiency, making it a reliable technology for pharmaceutical manufacturers.
FAQ
Why is Wiped Film Molecular Distillation preferred for heat-sensitive pharmaceuticals?
It operates under high vacuum, lowering boiling points and allowing purification at low temperatures. This prevents degradation of heat-sensitive APIs, peptides, and natural compounds.
Can Wiped Film Molecular Distillation remove all residual solvents in pharmaceuticals?
It effectively removes most organic solvents, reducing levels to below regulatory limits (often <10 ppm). Its high vacuum and short path design ensure efficient solvent removal without damaging APIs.
Is Wiped Film Molecular Distillation suitable for chiral drug separation?
Yes, it separates chiral compounds based on subtle differences in volatility and molecular weight, even when their boiling points are very similar. This avoids the need for expensive chiral chromatography.
How does Wiped Film Molecular Distillation support lipid-based drug delivery systems?
It purifies lipid nanoparticles and emulsions by removing excess lipids or solvents at low temperatures, preserving their structure and functionality—critical for drug stability and bioavailability.
Does Wiped Film Molecular Distillation comply with pharmaceutical regulations?
Yes, it meets strict standards for purity, consistency, and safety set by agencies like the FDA and EMA. Its ability to produce high-purity products and log process data supports regulatory compliance.
Table of Contents
- Purification of Active Pharmaceutical Ingredients (APIs)
- Separation of Chiral Compounds
- Removal of Residual Solvents
- Concentration of Heat-Sensitive Bioactive Compounds
- Purification of Lipid-Based Drug Delivery Systems
- Deodorization and Decontamination of Pharmaceutical Excipients
- Scale-Up from Lab to Production
-
FAQ
- Why is Wiped Film Molecular Distillation preferred for heat-sensitive pharmaceuticals?
- Can Wiped Film Molecular Distillation remove all residual solvents in pharmaceuticals?
- Is Wiped Film Molecular Distillation suitable for chiral drug separation?
- How does Wiped Film Molecular Distillation support lipid-based drug delivery systems?
- Does Wiped Film Molecular Distillation comply with pharmaceutical regulations?

